
Smart Technology
Better Solutions
Technology in the right hands leads solutions to success.
We at SCOPE believe in technological solutions that support the way we and our customers work. Our goal is to be flexible to set up integrations and to build new systems and features as the need arises. Consequently, we built our own solutions based on a proven platform.
As a midsize Contract Research Organization (CRO), we pride ourselves on leveraging cutting-edge technology to enhance the efficiency, accuracy, and overall quality of clinical trials. Below, you will find detailed information about our key technological solutions and how they support our mission to deliver excellence in clinical research.



We are your partner for study specific solutions that exactly meet your needs
Artificial Intelligence
Advancing Possibilities with AI
At the heart of our company’s mission is a dedication to innovation, ensuring that advancements are pursued where they offer real value rather than for their own sake. This approach is particularly evident in our adoption of Artificial Intelligence (AI) across the organization, which is guided by comprehensive risk-benefit analyses, regulatory compliance, and a strong commitment to scientific integrity. Above all, protecting our client data is our highest priority; we apply measures to ensure that client information remains private and secure at every step of our AI journey.
Our AI initiatives are purposefully designed to support, not replace, scientific and regulatory rigor. We are steadfast in our commitment to subject safety, data integrity, and compliance across every function we touch. We carefully select each AI tool to ensure it is appropriately suited to its intended purpose—much like choosing the correct instrument for a specific task, as one would not use a tractor to plant a tomato. Selecting the right AI solution is essential for effectiveness and precision.
Our AI initiatives are purposefully designed to support, not replace, scientific and regulatory rigor. We are steadfast in our commitment to subject safety, data integrity, and compliance across every function we touch. We carefully select each AI tool to ensure it is appropriately suited to its intended purpose—much like choosing the correct instrument for a specific task, as one would not use a tractor to plant a tomato. Selecting the right AI solution is essential for effectiveness and precision.
The AI Expert Group: Pioneering the Future
Our dedicated AI Expert Group stands at the forefront of this transformation. This cross-functional team actively scouts for emerging AI technologies, rigorously tests new functionalities, and collaborates closely with our subject matter experts across departments. By identifying and evaluating cutting-edge AI use cases (see below), our experts ensure we remain ahead of the innovation curve.
Where Thoughtfully Applied AI Enhances Research Value
- Study Feasibility: Predictive analytics and real-world data modeling to accelerate site selection and patient recruitment strategies.
- End-to-End Data Stewardship: From defining key data elements to managing validation, integration, and transformation – AI ensures consistency, not complexity.
- Medical Oversight Support: AI assists in planning targeted medical review by highlighting safety signals and suggesting alternative review strategies—enabling efficient, scientifically sound data oversight while preserving expert-driven decisions
- Medical Coding Automation: Machine learning technologies enhance auto coding capabilities by intelligently mapping clinical terms to standardized medical dictionaries, boosting accuracy, consistency, and coding efficiency across diverse datasets, particularly in large-scale or multilingual study environments.
- Medical Writing: Intelligent matching algorithms and Generative Pre-trained Transformer AIs assist with document design and generation, translation, and adaptation of text for different target audiences
- System Validation Optimization: Implement intelligent test planning, scenario development, and comprehensive documentation to improve validation efficiency while maintaining full compliance.
- Case Narrative Drafting: Language models accelerate clinical writing workflows, supporting—but not replacing—scientific judgment.
- Predictive Intelligence Targeted forecasting tools provide scenario planning and operational insights when backed by high-quality data.
Partnering for Progress
We recognise that genuine innovation may be achieved through collaboration. Accordingly, we are partnering with leading AI providers to ensure our clients gain access to the most current advancements in artificial intelligence.
Looking Forward
By leveraging AI, we enable smarter, more agile operations, helping our clients achieve their goals with confidence. Together, we are shaping a future built on intelligence and collaboration.
CTMS
Comprehensive Clinical Trial
Management System
INSIGHT is our own proprietary, powerful, web-based Clinical Trial Management System (CTMS) designed to consolidate, standardize, and visualize operational and clinical data from multiple sources. INSIGHT supports you with better and faster decision-making throughout the life of a trial.
Key features of our CTMS:
- Project Tracking & Visualization
- Protocol Deviation Management
- Data Import of subject recruitment data & programmatically detected protocol deviations
- Template Management
- Review & Approval Workflows
- Electronic Signature
- eTMF Integration
- Collaboration Tools
- Configuration & Customization
- Project Planning
- Global Contact Database
- Technical Support
- Quality & Regulatory Compliance
For detailed information about the CTMS functionality, kindly refer to our Factsheet (LINK)
eTMF
Electronic Trial Master File (eTMF)
At SCOPE, we are experienced with various eTMF providers including TransPerfect, Veeva, Medidata and Phlexglobal. Our preferred eTMF solution is Trial Interactive from TransPerfect, as it meets regulatory requirements at a fair market value. The partnership between TransPerfect and SCOPE is providing significant advantages to sponsors, including quicker set-up times for new trials, reduced initial costs, and more efficient file management through an Application Programming Interface (API) with SCOPE’s proprietary CTMS INSIGHT. These improvements have not only ensured the timely management of Trial Master Files but have also optimized our budget for TMF maintenance, demonstrating our commitment to leveraging innovative solutions for more cost efficient and effective clinical trial management.
Key Features of our eTMF Solution:
- Optimised costs: Study-specific eTMF rooms are built from an existing configuration (clone room) which is continuously developing while eTMF technology advances. The clone room provides minimal set-up costs for sponsor while including years of TMF experience. The API between the eTMF and CTMS allows for efficient filing and minimised budgets for TMF maintenance.
- Quick set-up times: The existing TMF configuration enables TMF go-life within weeks while requiring minimal sponsor involvement.
- TMF Reference Model: The existing TMF configuration is based on TMF Reference Model.
- Inspection Readiness: The API between the eTMF and CTMS in combination with SCOPE’s TMF Plan ensures inspection readiness at all times. Unlike usual TMF completeness checks which compare against manually created events and placeholders, SCOPE’s TMF completeness is assessed against the entirety of all documents in SCOPE’s CTMS INSIGHT, thereby providing a more relevant TMF expectation at reduced efforts and costs.
EDC / ePRO /
RTMS
RTMS
Electronic Data Capture (EDC) and Electronic Patient-Reported Outcomes (ePRO)
SCOPE works with a range of EDC and ePRO vendors to provide flexible and reliable data capture solutions. Our partnerships with vendors like Viedoc, Merative and Oracle ensure that we can adapt to the preferred systems of our clients, offering seamless integration and high-quality data management.
Key Features of our EDC and ePRO Solutions:
- Experienced Team: Our team has extensive experience with different EDC systems and has been building studies for many years, making individual adaptations to the EDC possible fast
- Customizable Solutions: We offer customizable solutions to meet the specific needs of each study, ensuring high-quality data capture and management
- Integration with ePRO: We consolidate data from all sources and vendors into a harmonized dataset, that supports confident decision-making.
Automated Safety Reporting
Automated Safety Reporting
SCORIS (SCOPE Reporting Information System) delivers a fail-safe reporting function with automated safety reporting and tracking to ensure all national safety reporting requirements are met for all key stakeholders, such as regulatory bodies and investigators.
Key features of our Safety Reporting Solution:
- Automated safety reporting: Including automated safety report tracking (audit trail)
- Tracking and scheduling of national and local safety reporting requirements for all countries: Ensures reporting to ethics committees (ECs), regulatory authorities (RAs), investigators, marketing authorization holders, etc.
- Safety reporting contact details: Tracking and review of all (ECs, RAs, local authorities, investigators, MA holder); information available across studies
- Timeline management: Study approval and start dates as well as study end dates for setting reporting timelines at country level
- Independent master safety database: Allows for storing safety reporting data
Customized Solutions
Customized Solutions
Regardless of the dimensions of your study, we can provide the technical solution that works for you. In the last years, we have successfully set up and used study specific solutions like a Subject Randomization System and a study specific IP Allocation System.
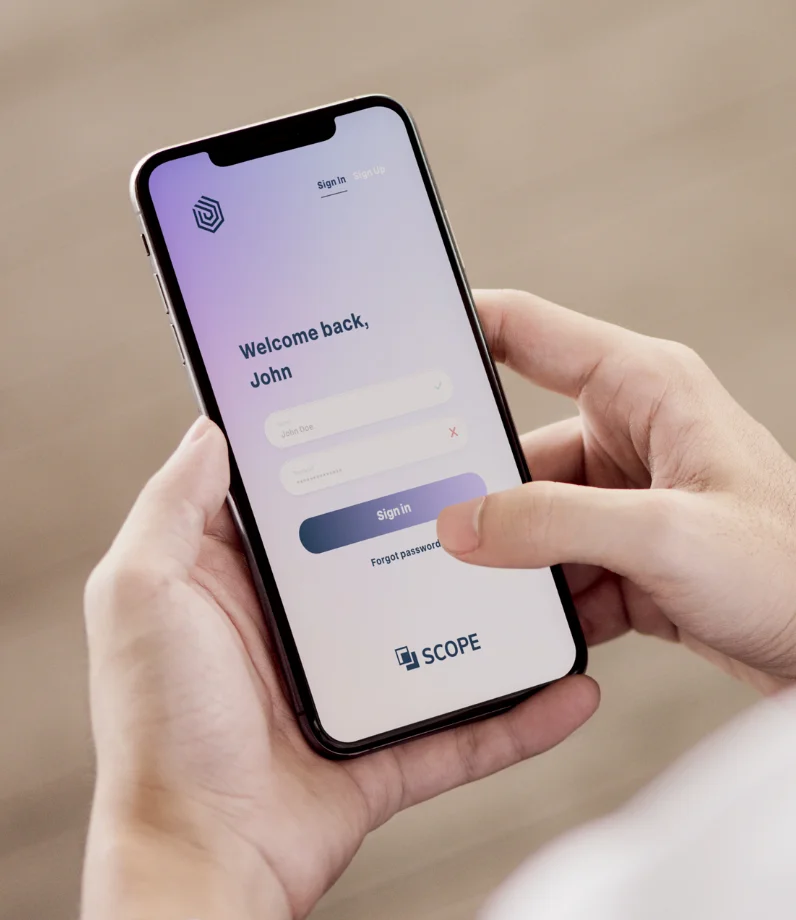
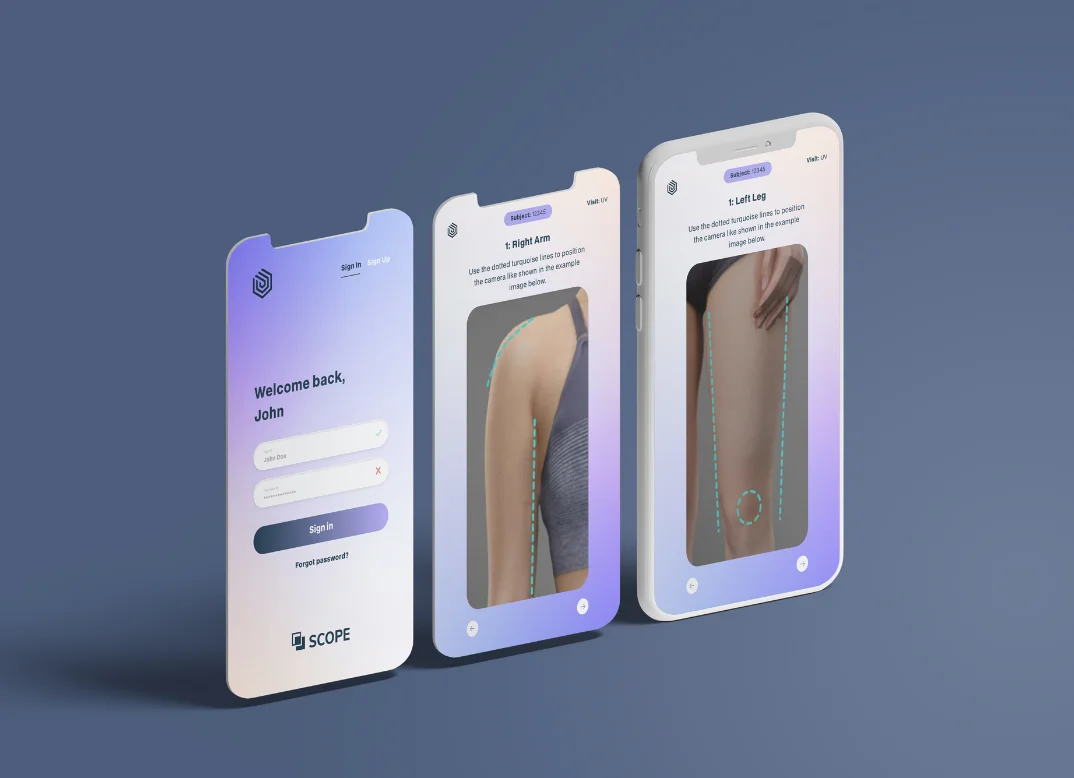
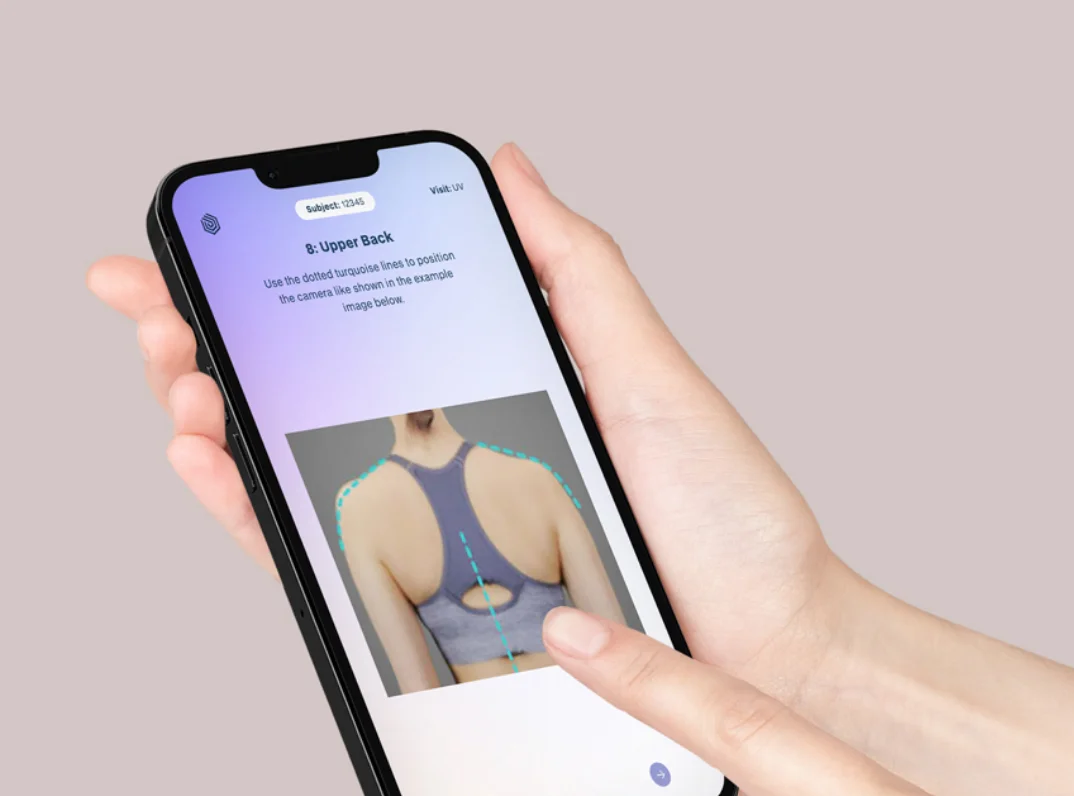
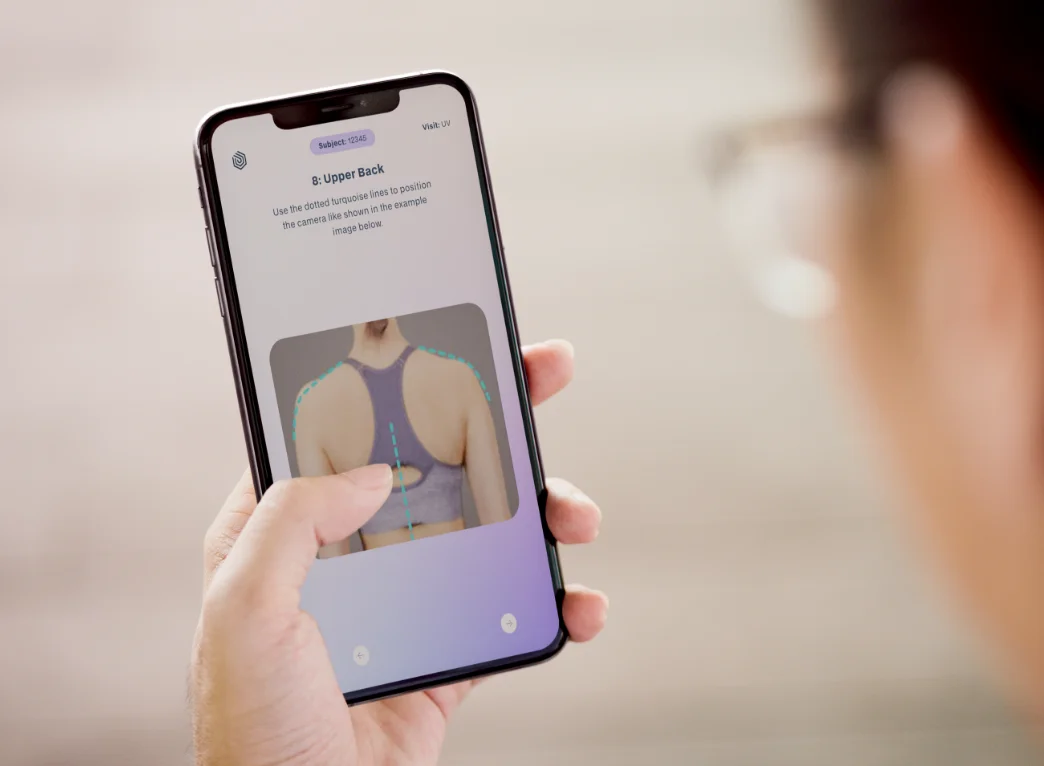
Our most recent opus, a custom mobile app, was developed to document evidence in a study in the dermatological area with ease. Investigators across 5 countries acquired sets of photographs via mobile phones by following simple user guidance and transferred it safely to a central storage. Concept, Requirements Management, Risk Assessment, Implementation and Validation of the Custom Computerized System according to life science regulations were performed by SCOPE. The App was strongly tailored to the needs of the Sponsor – in time and budget with proven quality. You don‘t need to worry about user support, platform maintenance, product improvement and data archival – SCOPE covers the full product lifecycle.
Our most recent opus, a custom mobile app, was developed to document evidence in a study in the dermatological area with ease. Investigators across 5 countries acquired sets of photographs via mobile phones by following simple user guidance and transferred it safely to a central storage. Concept, Requirements Management, Risk Assessment, Implementation and Validation of the Custom Computerized System according to life science regulations were performed by SCOPE. The App was strongly tailored to the needs of the Sponsor – in time and budget with proven quality. You don‘t need to worry about user support, platform maintenance, product improvement and data archival – SCOPE covers the full product lifecycle.
eConsent
eConsent – Modernizing Informed Consent Through Trusted Partnerships
At SCOPE, we recognize that informed consent is a critical foundation of ethical clinical research. To enhance patient understanding, engagement, and regulatory compliance, we offer eConsent solutions through collaboration with leading technology vendors in the field. By partnering with specialized providers, we ensure that our clients benefit from state-of-the-art platforms that support multimedia content, electronic signatures, and real-time access—while maintaining full compliance with global regulatory standards.
Why We Use eConsent via Trusted Vendors:
- Enhanced Patient Comprehension through interactive and visual content
- Remote Access to consent materials on any device
- Streamlined Documentation with audit trails and version control
- Multilingual Capabilities for global trial deployment
- Regulatory Compliance with GDPR, 21 CFR Part 11, and local ethics requirements
- Seamless Integration with EDC, ePRO, and CTMS systems
Our vendor-based eConsent approach allows us to remain flexible and scalable —tailoring the solution to each study’s needs while ensuring a consistent, patient-centric experience.
Contact
Let`s grow together!
SCOPE International AG
Konrad-Zuse-Ring 18
68163 Mannheim, Germany
+49 621 429-390
contact@scope-international.com
contact usKonrad-Zuse-Ring 18
68163 Mannheim, Germany
+49 621 429-390
contact@scope-international.com

